The Next Frontier
of Immuno-Oncology
Botensilimab (Fc-enhanced anti-CTLA-4) plus Balstilimab (PD-1 inhibitor)—is a next-generation immunotherapy combination in phase 3 clinical development. It activates the immune system to recognize and eliminate cancer—including tumors that have failed prior treatments.
Mechanism of Action
How BOT + BAL Work Together
BOT+BAL combines two powerful antibodies designed to work together to activate the immune system against cancer:
A next-generation Fc-enhanced multi-functional anti-CTLA-4 antibody “primes” the immune system. BOT helps activate new cancer-fighting T cells, remove suppressive immune cells that block response, and strengthen long-term immune memory.
Botensilimab
An anti-PD-1 antibody that keeps those activated immune cells engaged and prevents them from shutting down too soon.
Balstilimab
Built for Broader and More Durable Benefit
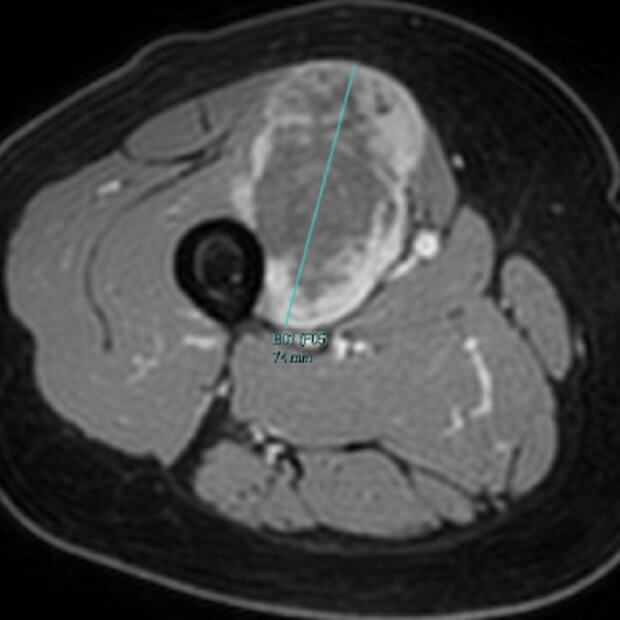
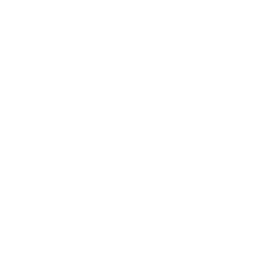
BOT, alone or in combination with BAL, has been has been evaluated in over 1,200 patients across nine tumor types—including colorectal, sarcoma, ovarian, breast, liver (hepatocellular), lung (NSCLC), and melanoma cancers—in neoadjuvant, first-line, and late-line refractory settings.

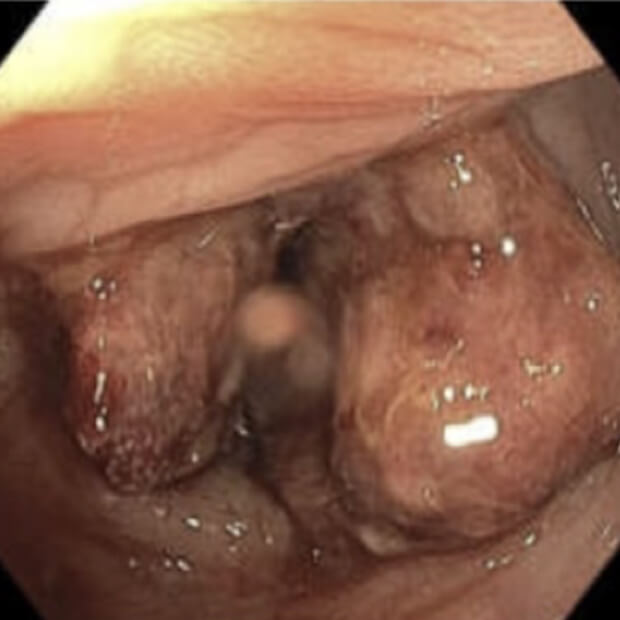
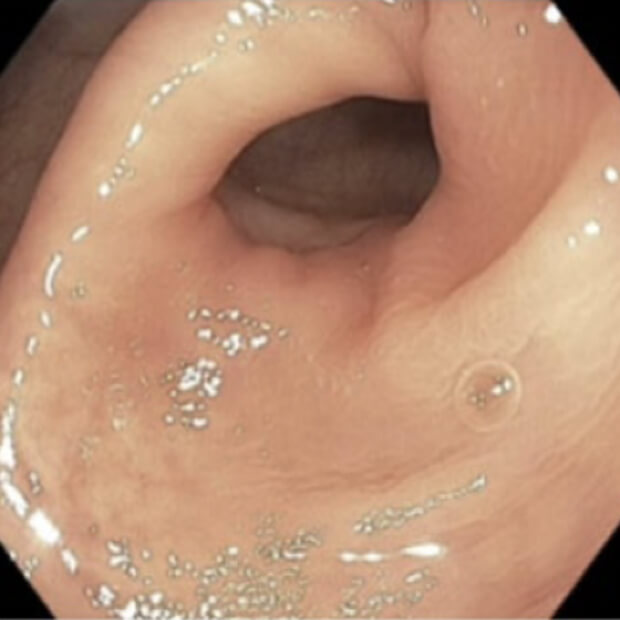
Turning “Cold” Tumors → “Hot”
Together, BOT plus BAL are designed to convert “cold” tumors—those that have been historically unresponsive to earlier immunotherapies—into “hot,” immune-active ones that can be targeted and destroyed.
“Cold” tumors are filled with cells that block or quiet the immune response. Because the immune system can’t see or attack these tumors, they often do not respond well to immunotherapy.
“Hot” tumors are different. They contain active immune cells—like T cells and NK cells—that can recognize and attack cancer. By turning cold tumors hot, BOT + BAL make tumors more likely to respond to treatment.
Read the Science
More Powerful
More Durable
More Human
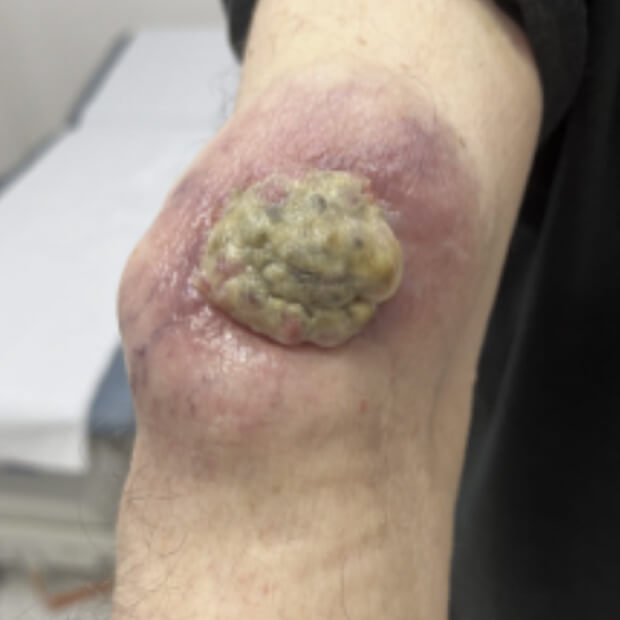
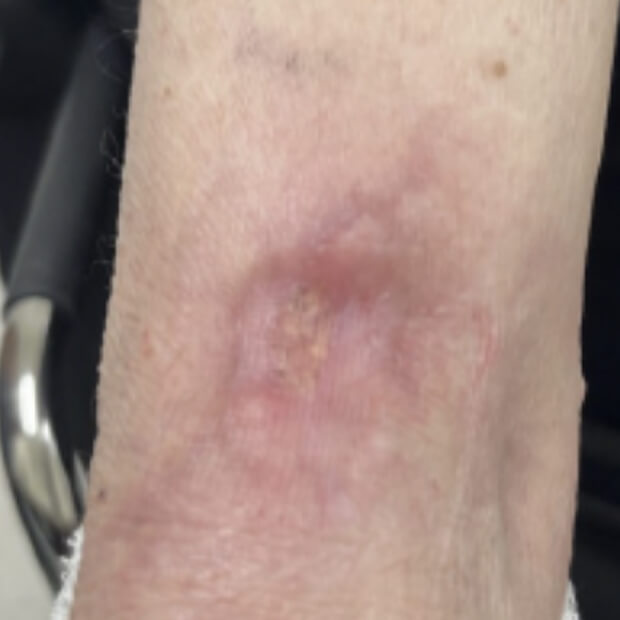
The combination of botensilimab (BOT) and balstilimab (BAL) reprogram immune-resistant tumors, driving meaningful and durable responses where immunotherapy has historically failed.

Our Commitment
Enabling Access to Medicines
Agenus is committed to making our investigational medicines available to patients with cancer. Our goal is to provide access to our investigational medicines at the appropriate time and in the correct manner for patients.
Why Immuno-Oncology?
The Science of Surviving & Thriving
Traditional cancer treatments like chemotherapy and radiation attack both healthy and cancer cells. Immunotherapy works differently—it helps the body do what it was built to do: recognize, attack, and remember cancer. BOT+BAL represents the next evolution of that mission.
Questions?
Do you have any questions about Agenus research and development? Feel free to reach out.