Using Your Immune System to Defeat Cancer

Innovative Immunotherapies
At Agenus, we’re reimagining cancer treatment by activating the immune system to do what it was meant to do—recognize and eliminate disease. Our comprehensive portfolio of immunotherapies works across the cancer immunity cycle, with the goal of reducing reliance on chemotherapy, radiation, and invasive surgeries—helping more patients live longer, fuller lives.
Learn how our science is changing what’s possible
“30 years ago, we envisioned a future where the immune system could be harnessed to cure cancer. That vision remains our driving force today—science-led, patient-focused, and unwavering in our mission to transform outcomes.”
BOT + BAL
Bringing the Power of Immunotherapy to More Patients
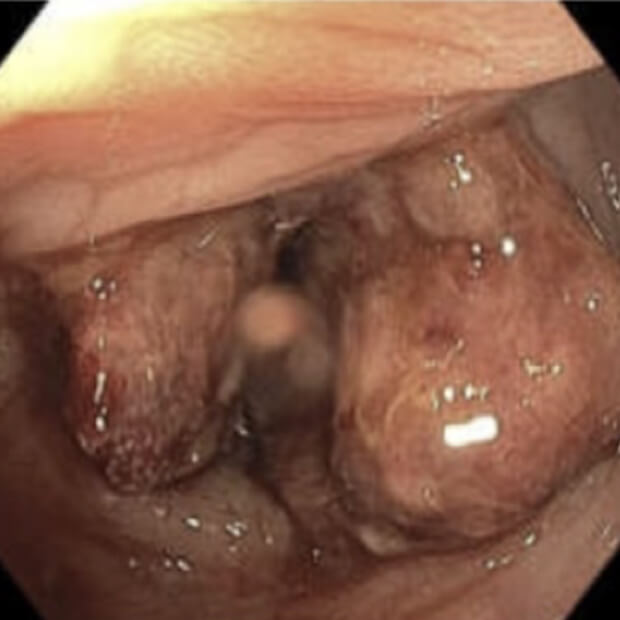
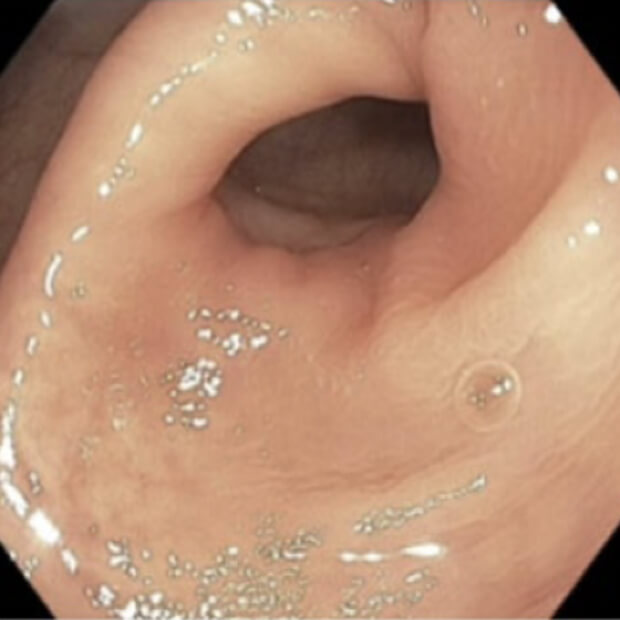
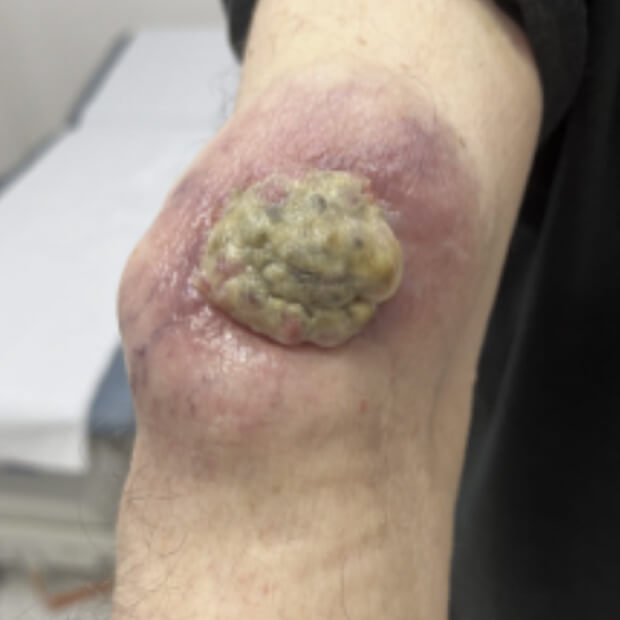
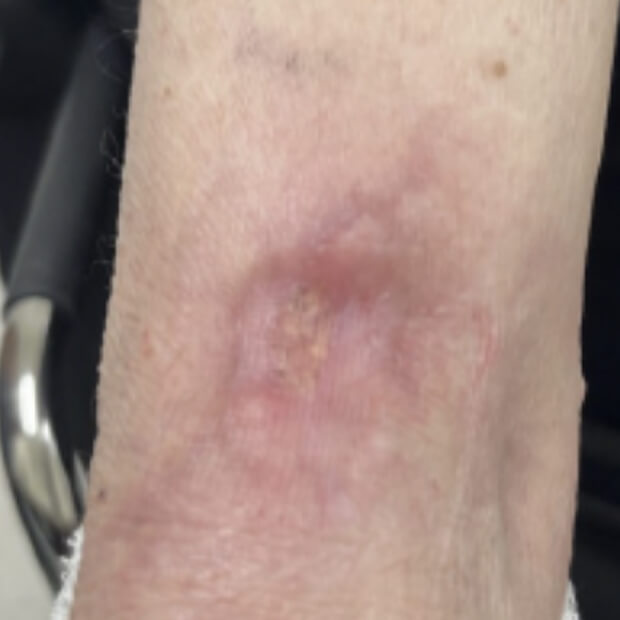
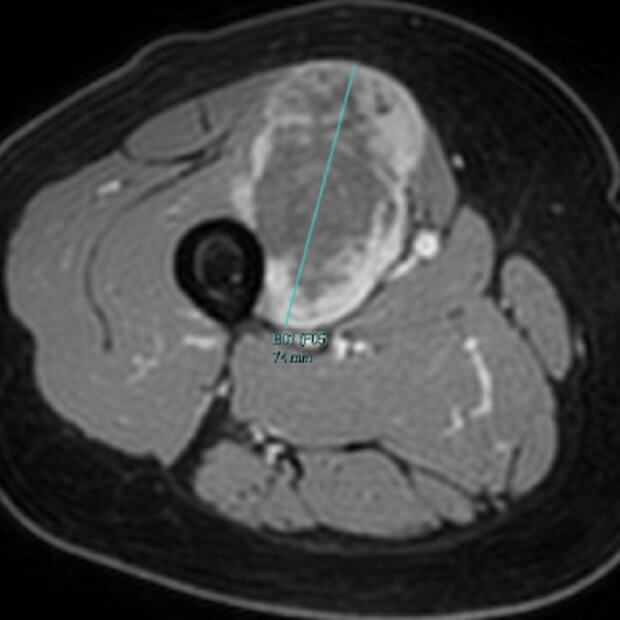
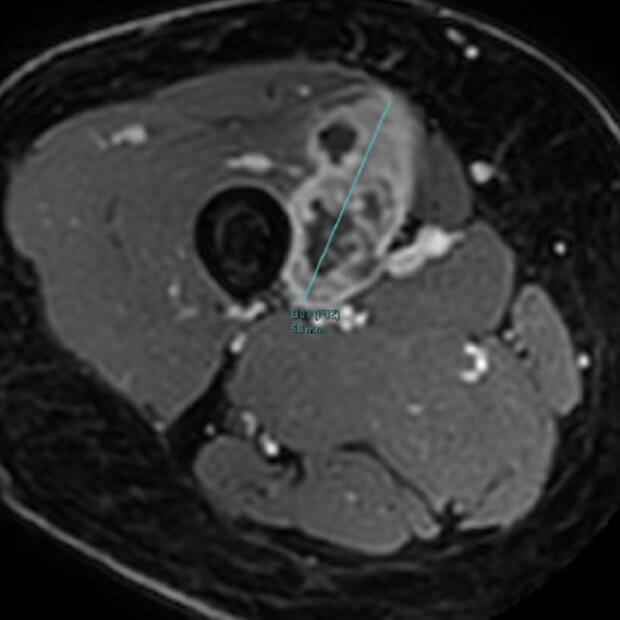
Botensilimab (BOT) plus Balstilimab (BAL) represents the next generation of immunotherapy, designed to help more patients benefit from the body’s own defenses against cancer.
In the News
Get answers about clinical trials, patient access, and more

Acceleration Through Partnerships
Agenus advances its mission through strategic collaborations that expand the reach and impact of our science. While we retain ownership of most programs, select partnerships bring global development expertise, capital, and technology that accelerate discovery, strengthen our pipeline, and improve patient outcomes.



By the Numbers
Our vision is to broaden the number of patients who benefit from immunotherapy by advancing combination approaches that draw on our diverse portfolio of antibody therapeutics, access to adoptive cell therapies, and adjuvant and vaccine platforms.